

These Lewis acid-base adducts are exemplified by HCNKrF+ and F3CCNKrF+, which are formed as AsF6− salts. The KrF+ cation has been shown to behave as a Lewis acid (electron-pair acceptor) toward a number of Lewis bases that are resistant to oxidation by the strongly oxidizing KrF+ cation at low temperatures. The KrF+ cation behaves as only an oxidizing agent in converting gaseous oxygen to O2+. The KrF+ cation ranks among the most powerful chemical oxidizers presently known and is capable of oxidative fluorination of gaseous xenon to XeF5+ and chlorine, bromine, and iodine pentafluorides to the ClF6+, BrF6+, and IF6+ cations, respectively. The Kr2F3+ cation is V-shaped with a fluorine atom bonded to each of two krypton atoms and both krypton atoms bonded to a common fluorine in the middle, i.e., F(KrF)2+. The cationic species KrF+ and Kr2F3+ are formed in reactions of KrF2 with strong fluoride-ion acceptors such as the pentafluorides of Group 15, in which the fluoride ion F− is transferred to the pentafluoride to give complex salts that are analogous to those of XeF2 here no oxidation is involved. Hence, in a formal sense, oxidative fluorination is the net result of extraction of two electrons and addition of F− this can be considered to be equivalent to the transfer of F+.) KrF2 is, for example, capable of oxidizing and fluorinating xenon to XeF6 and gold to AuF5.
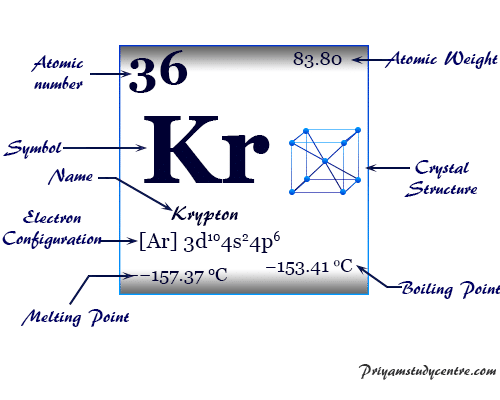
Its fluorinating ability means that it transfers an F− ion to other substances. (Its oxidizing power means that it extracts electrons from other substances and confers on them a positive charge. Krypton difluoride is a powerful oxidative fluorinating agent. No other molecular fluoride of krypton has been isolated, so all krypton compounds are derived from KrF2, where Kr is in the +2 oxidation state. KrF2 is a colourless crystalline solid that is highly volatile and slowly decomposes at room temperature. Several other methods for the synthesis of KrF2 are now known, including irradiation of krypton and fluorine mixtures with ultraviolet radiation at −196 ☌ (−321 ☏). In the early 1960s, however, krypton was found to react with the element fluorine when both are combined in an electrical-discharge tube at −183 ☌ (−297 ☏) the compound formed is krypton difluoride, KrF2. For many years it was considered to be totally unreactive. Krypton is the lightest of the noble gases that form isolable chemical compounds in macroscopic amounts. After it has been stored a few days, krypton obtained by nuclear fission contains only one radioactive isotope, krypton-85, which has a half-life of 10.7 years, because all the other radioactive isotopes have half-lives of 3 hours or less. The longest-lived of these, krypton-81, has a half-life of 229,000 years. Krypton has isotopes of every mass number from 69 through 101 of these isotopes,25 are radioactive and are produced by fission of uranium and by other nuclear reactions.

Natural krypton is a mixture of six stable isotopes: krypton-84 (56.99 percent), krypton-86 (17.28 percent), krypton-82 (11.59 percent), krypton-83 (11.5 percent), krypton-80 (2.29 percent), and krypton-78 (0.36 percent). (One metre equaled 1,650,763.73 times the wavelength of this line.) The wavelength of an orange-red component of light emitted by stable krypton-86, because of its extreme sharpness, served from 1960 to 1983 as the international standard for the metre. When a current of electricity is passed through a glass tube containing krypton at low pressure, a bluish white light is emitted. Krypton is named from the Greek word kryptos, “hidden.” Radioactive krypton-85 is useful for detecting leaks in sealed containers, with the escaping atoms detected by means of their radiation. Krypton is used in certain electric and fluorescent lamps and in a flashlamp employed in high-speed photography. Travers in the residue left after a sample of liquid air had boiled almost entirely away. The element was discovered in 1898 by the British chemists Sir William Ramsay and Morris W. Although traces are present in meteorites and minerals, krypton is more plentiful in Earth’s atmosphere, which contains 1.14 parts per million by volume of krypton. About three times heavier than air, krypton is colourless, odourless, tasteless, and monatomic. Krypton (Kr), chemical element, a rare gas of Group 18 (noble gases) of the periodic table, which forms relatively few chemical compounds. Key People: Sir William Ramsay Related Topics: chemical element noble gas air krypton-81 Our editors will review what you’ve submitted and determine whether to revise the article.


 0 kommentar(er)
0 kommentar(er)
